Primodos: The first step towards Justice
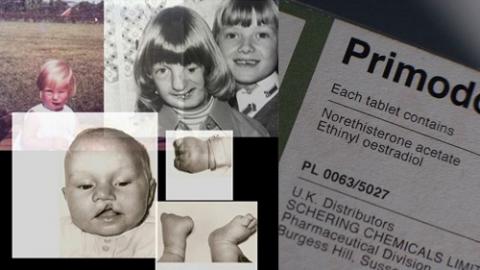
Image Source: Primodos: Sky News exposes pregnancy drug cover-up
Sharon Hartles was awarded an MA in Crime and Justice (with distinction) from The Open University and is a member of the Harm and Evidence Research Collaborative (HERC).
Primodos was the most widely used hormone pregnancy test prescribed to women in the UK. During 1958 to 1970 Primodos was marketed as a hormone pregnancy test and for the treatment of secondary amenorrhea. However, this was changed to just the treatment of secondary amenorrhea from 1970 to 1978, at which stage Primodos was withdrawn from the UK market. When Primodos was placed on the UK market in 1958 there was no centralised structured pharmaceutical regulation. In other words, no licence was required, no specific safety test was needed and there was no general consumer protection legislation.
In 1978, the Association for Children Damaged by Hormone Pregnancy Tests, was set up in the UK to represent families who suffered congenital abnormalities, stillbirths and miscarriages, allegedly due to taking the oral hormone pregnancy test Primodos. Decades of fighting for justice to uncover the truth about the failures of past Government Health Regulatory Authorities led to a review being commissioned in February 2018, by Jeremy Hunt, the then, Secretary of State.
The announcement in the House of Commons was for a review into how the health system responds to reports about harmful side effects from medicines. This stemmed from patient-led activist campaigns on the use of: hormone pregnancy test Primodos, sodium valproate and surgical mesh. Jeremy Hunt stated “patients and their families have had to spend too much time and energy campaigning for answers in a way that has added insult to injury for many.”
Two and a half years after this review was commissioned, on Wednesday 8th July 2020, the Independent Medicines and Medical Devices Safety Review published the First Do No Harm Report. This Report, together with the additional supporting documents to accompany it including: Personal Testimonies, Oral Hearing Transcripts, Hormone Pregnancy Tests Supporting Information, Timeline Key Events, History of Regulation and the Press Conference Speech (by Baroness Julia Cumberledge, CBE, Chair of the Independent Medicines and Medical Devices Safety Review) evidence unequivocal systemic failures and a clear link between PRIMODOS and its tragic side-effects.
Marie Lyon, Chair of the Association for Children Damaged by Hormone Pregnancy Tests and active campaigner for justice, since 1978, on reading the First Do No Harm Report, declared “I’ve tried to be very calm and I can’t. It’s the fact it’s been acknowledged. They’ve actually looked at the documentation honestly and openly and for me that is the biggest result for our families today. They will be absolutely overjoyed.”
The Independent Medicines and Medical Devices Safety Review has set out nine recommendations in their First Do No Harm Report. Recommendation 1: states ‘The Government should immediately issue a fulsome apology on behalf of the healthcare system to the families affected by Primodos, sodium valproate and pelvic mesh.’ On the 8th July (the date the report was published) Matt Hancock, Secretary of State for Health and Social Care apologised “on behalf of the NHS and the whole healthcare system” to those who have suffered and their families.
For decades, there have been numerous publications evidencing an association between hormone pregnancy tests and congenital malformations in babies. In 2018 and 2019, Oxford University published an analysis of data which found a clear association relating to Primodos and birth defects. Other supporting research which have found links between hormone pregnancy tests and birth defects includes:
- Congenital limb reduction deformities and use of oral contraceptives (1986)
- Maternal drug histories and congenital abnormalities (1977)
- Congenital heart disease and prenatal exposure to exogenous sex hormones (1977)
- Hormonal Pregnancy Tests and Congenital Malformation (1967)
However, there have also been opposing publications which have found no association and/or inconclusive results. In 2017, the Medicines and Healthcare products Regulatory Agency (MHRA) published their report on the use of hormone pregnancy tests and adverse effects related to pregnancy including possible birth defects. The MHRA is an independent Expert Working Group of the UK’s commission on Human Medicines, which was established, in October 2015, in order to conduct this review. The MHRA found there to be insufficient evidence to support an association. Other opposing research includes:
- Congenital bladder exstrophy associated with Duogynon hormonal pregnancy tests—Signal for teratogenicity or consumer report bias? (2014)
- Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations (1983)
- Hormonal Pregnancy Tests and Congenital Malformations (1973)
For Marie Lyon, Chair of the Association for Children Damaged by Hormone Pregnancy Tests “after viewing the oral evidence presented by members of the Expert Working Group who were responsible for the scientific publication in 2017, it seems I already have a perfect example of the denial and protection culture endemic in our regulators. Denial when problems occur and protection, not for the patient but for the manufacturer.”
In light of the decades of jostling to and fro of supporting and opposing evidence, it is clearer to understand why the findings of the Independent Medicines and Medical Devices Safety Review in the First Do No Harm Report, together with Matt Hancock’s prompt apology on behalf of the UK Government and acceptance may in the first instance offer some form of relief for the families of the Association for Children Damaged by Hormone Pregnancy Tests.

In the Press Conference Speech by Baroness Julia Cumberledge Chair of the Independent Medicines and Medical Devices Safety Review, she stated ‘In our view Primodos continued to be given as a pregnancy test for years longer than it should. In the face of growing concerns it should have ceased to be available from 1967.’ Yet Primodos remained on the UK market until 1978. This is a failure on behalf of the UK Government to protect its population from harm. Equally, a failure on behalf of the corporation Bayer (Schering). Primodos, was manufactured by Schering in Germany. In 2006 Schering was acquired by Bayer plc.
It is important to point out that Amenorone Forte a hormone pregnancy test prescribed by GPs, during this same time frame, acted in much the same way as Primodos and was manufactured by Roussel in France. Roussel was acquired by Sanofi in 2004. For this reason families of the Association for Children Damaged by Hormone Pregnancy Tests hold both corporations accountable for the avoidable harm inflicted.
According to the Independent Medicines and Medical Devices Safety Review, History of Regulation, The Medicines Act 1968 received Royal Assent in October 1968, however the ‘transitional period’ meant this Act did not come into effect until 1st September 1971. During this time the Committee on Safety of Drugs was formed, yet it had no legal powers. With little irony, there was no formal regulator, it was part of a voluntary arrangement. There was no body to legally mandate the removal of a drug from the market and limited mechanisms to regulate drugs and restrict their use.
More systemic failures followed because the Committee on Safety of Medicines, (which replaced the Committee on Safety of Drugs, 1st September 1971) focused its gaze on formalising new medicines entering the UK market. Products, including Primodos, which had been on the market before the 1st September 1971 were automatically granted a Product Licences of Right (PLR).
Primodos was awarded a PLR yet its product which had been on the market since 1958, had never been required to submit evidence of quality, safety or efficacy. This oversight to ensure Primodos met the appropriate standards of safety, quality and performance in line with new rules was another missed opportunity to protect public health and safeguard the interests of patients and users.
The Independent Medicines and Medical Devices Safety Review Timeline has brought to light other damning evidence. On 22nd July 1969 Schering UK wrote to Schering Germany recommending the removal of the pregnancy testing indication. In a letter dated 17th February 1970 to Schering, Dr Ruttle a member of the Standing Committee on the Classification of Proprietary Preparations (known as the MacGregor Committee – 1965 and 1971) which provided guidance as to which preparations should be used on the NHS, stated ‘The Committee would be prepared to place the product in A.3 if the promotional indication as a “pregnancy test” were withdrawn and I would suggest that the most appropriate and, acceptable to the Committee, promotion be “symptomatic treatment of amenorrhea to produce withdrawal bleeding.”
On the 9th March 1970 Schering agreed ‘to the deletion of “pregnancy test” from the indications, and to the promotional statement “the symptomatic treatment of amenorrhea not due to pregnancy, by producing withdrawal bleeding”. Further correspondence in April 1970 acknowledged the suggestions from Schering (removing the pregnancy test indication and altering promotional statements) and confirmed that Primodos would be placed in category A.3 (prescription-only medicines).
Five years later, the Committee on Safety of Medicines (an independent advisory committee to the UK medicines licencing authority) published a letter in the British Medical Journal (BMJ) on 26th April 1975. In this letter the Committee on Safety of Medicines stated they agreed with an article published five months earlier in the BMJ entitled Synthetic Sex Hormones and Infants which advised ‘there is little justification for the continued use of withdrawal type pregnancy tests when alternative methods are available.’
On 5th June 1975, the Committee on Safety of Medicines sent an alert letter – to all doctors in the UK – entitled Hormonal Pregnancy Tests, in which they advised them of a possible association between hormonal pregnancy tests and an increased incidence of congenital abnormalities. The Committee on Safety of Medicines stated ‘In view of the possible hazard, doctors should not normally prescribe certain hormonal preparations for pregnancy tests’.
Spanning 1958 to 1978, Primodos was given to around 1.5million women in Britain. Primodos was a hormone pregnancy test prescribed to women to detect pregnancy. It consisted of two tablets which were to be taken on consecutive days. A negative pregnancy test would result in a withdrawal bleed (within three to ten days of consumption of the tablets). It is now known that Primodos prescribed to women to confirm their pregnancy, by today’s standards equates to 13 morning-after pills or 40 oral contraceptive pills. Moreover, the hormones contained in Primodos are now used in the morning-after contraception pill.
A statement taken from the Independent Medicines and Medical Devices Safety Review Personal Testimonies from the families of the Association for Children Damaged by Hormone Pregnancy Tests illustrate their distress – “We feel that we were used as collateral damage by the pharmaceutical company who were developing the contraceptive drug at the time.” The personal testimonies of Nicky Gibbins and Daniel Mason evidence how “The effect on our lives have, as you can imagine, been devastating.” The alleged impacts of PRIMODOS comprise:

- all congenital malformations
- more specific malformations:
- cardiac malformations
- musculoskeletal
- neurological
- neurogenetical malformations
- birth defects
- miscarriage
- stillbirth
The First Do No Harm Report together with the supporting documents is significant because it evidences a clear link between Primodos and the terrible avoidable harms that have been perpetuated for decades through a culture of denial and the absence of state and corporate accountability.
Acknowledgement in the form of an apology on behalf of the Government was the first step towards justice. However, in a letter dated 13th December 2018, to the Independent Medicines and Medical Devices Safety Review, Bayer stated ‘there is nobody at Bayer plc who could usefully contribute anything on the subject matter of your inquiry’. Notwithstanding this response, it is now time to look to the future.
The Government (on behalf of the UK regulators) and corporations Bayer (Schering) and Sanofi (Roussel) should as recommended in the First Do No Harm Report, fund the costs of care for those affected by state and corporate harm. In addition to this, the families of the Association for Children Damaged by Hormone Pregnancy Tests using the Independent Medicines and Medical Devices Safety Review evidence should be able to successfully take legal action for the harms done to them by Bayer, Sanofi and the regulators.
In line with recommendation 9, of the First Do No Harm Report, the Government has a duty to set up a task force which must schedule a timeline for the implementation of the remainder of the recommendations. Such initiatives should endeavour to provide a safety net to ensure that a patient-led approach is centred at the heart of future health care provision.
